SITUATION
On November 11, 2020, the European Commission published a policy briefing on "Strengthening our medical and scientific agencies", under which a new EU Health Emergency Preparedness and Response Authority (EU HERA) is introduced. A conclusive proposal has been announced for late 2021. This kENUP discussion paper aims to contribute to the creation of an effective and efficient mechanism to fight future pandemics.
The current pandemic has revealed massive market failures in the development of interventions against pandemic tail risks. High development costs are coupled with low-profit potential, thus leading to a depressed supply of therapeutic and vaccine R&D when considering the probability for the occurrence of such a tail risk.
In response to the current pandemic, policy discussions in Europe are focusing on the creation of a European agency for late-stage biomedical research modelled on the US Biomedical Advanced Research and Development Authority (BARDA). In the US, BARDA, alongside the Department of Defense, is managing ‘Operation Warp Speed’, de-risking the rapid development of vaccines against COVID-19.
PROPOSAL
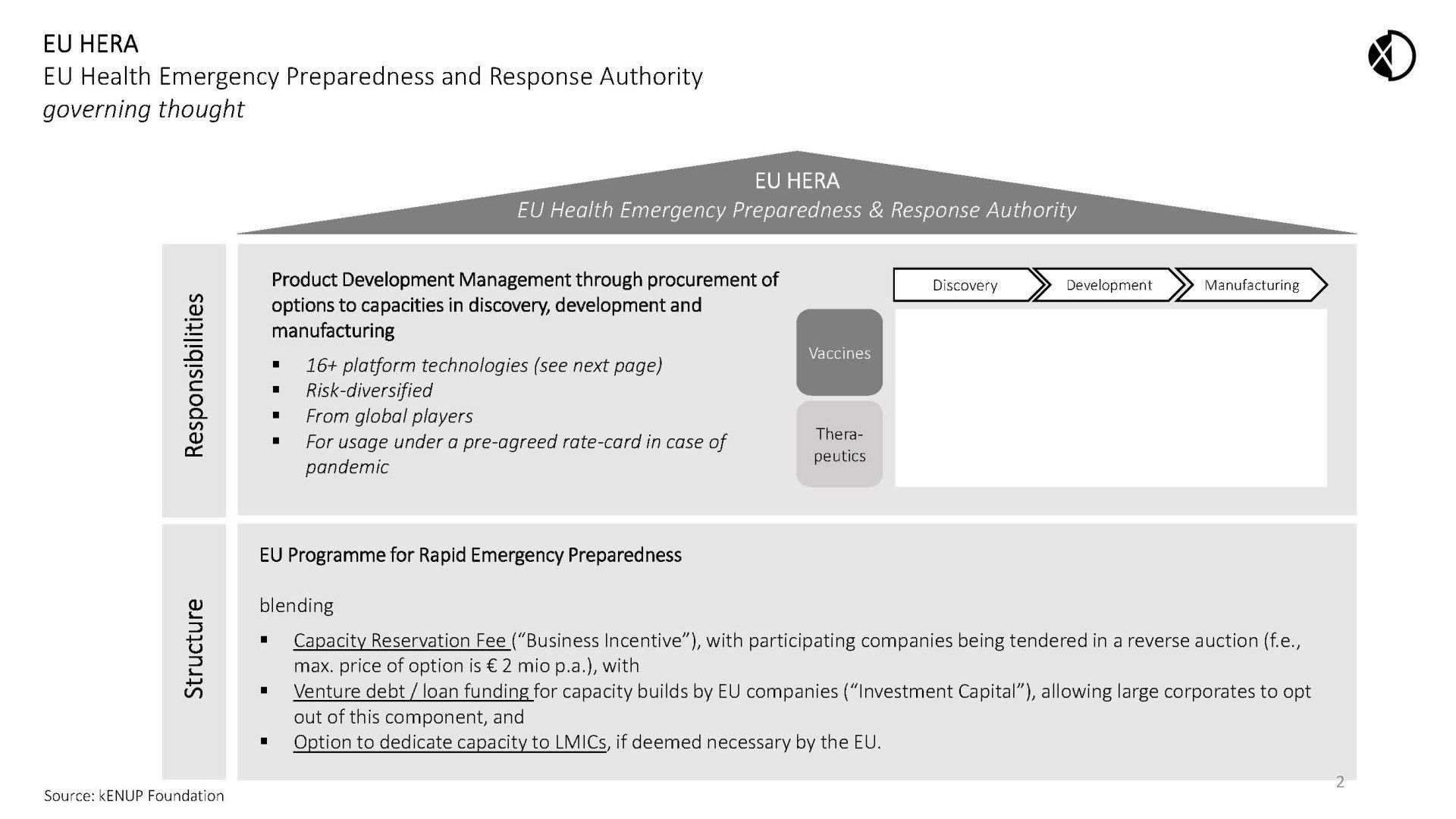
A coordinated European institutional approach to incentivize and accelerate preparedness for future pandemic pathogens is necessary to master this unprecedented challenge. kENUP Foundation proposes a dedicated EU Programme for Rapid Emergency Preparedness, overseen by EU HERA to
- incentivize the development of therapeutic and vaccine R&D against future potential pandemic pathogens;
- guarantee the EU access to R&D and production capacities at pre-agreed terms to swiftly protect Europeans and everyone else from new pathogens - during the last century, most dangerous outbreaks remained regional;
- boost EU’s globally leading companies active in biologicals;
- fairly share relevant resources with LMICs to prevent future local epidemics from turning into pandemics
STRUCTURE
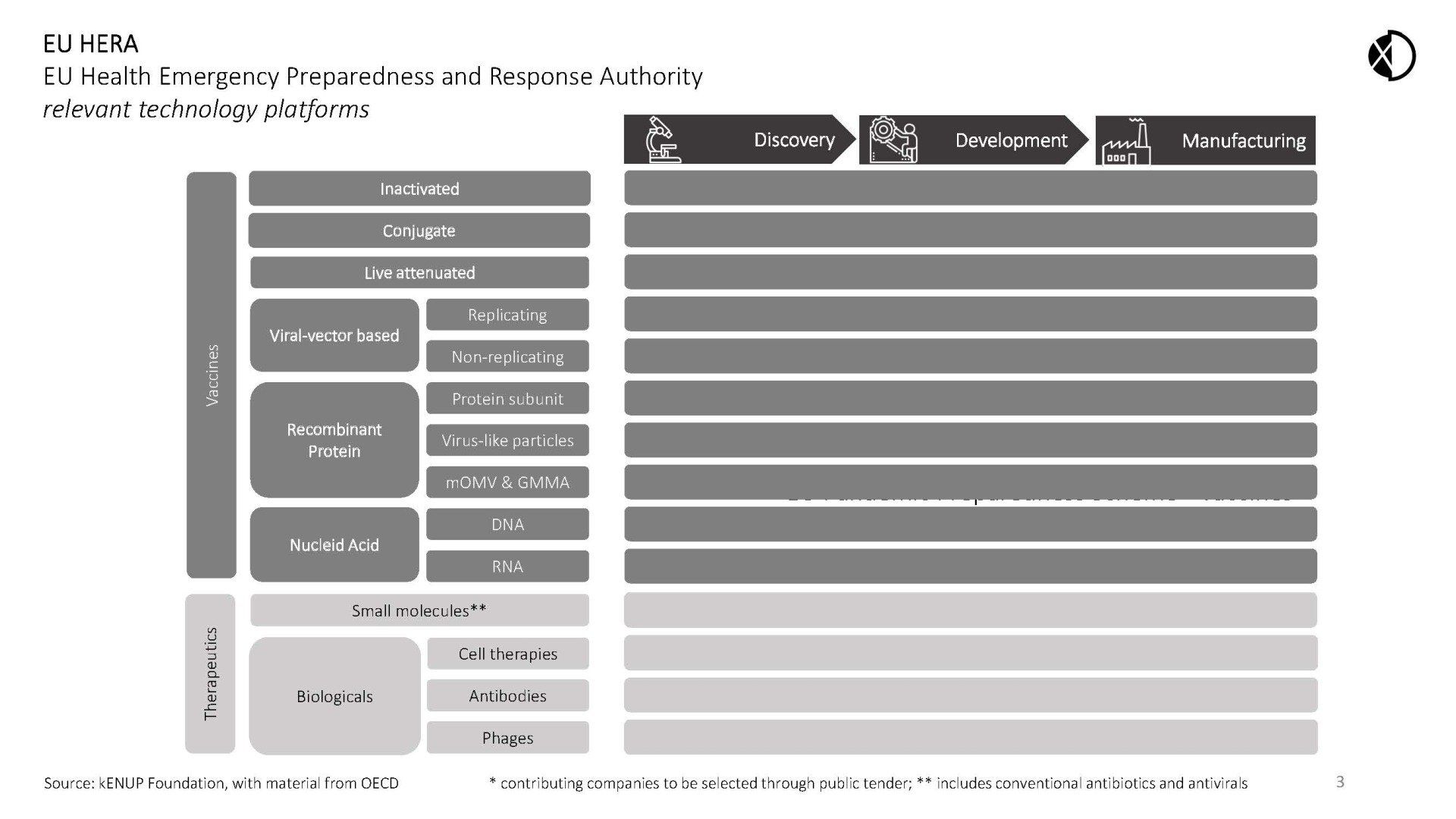
The programme aims to incentivise the development of essential products needed to fight a future pandemic through advance procurement of options to capacities on platform technologies relevant for the discovery, development and manufacturing of biologics.
Under the Programme, EU HERA would purchase guaranteed access to capacities instrumental to fight future pandemic pathogens at pre-agreed rates by paying an annual reservation fee to a selection of around 16+ platform companies (“Capacity Reservation Fee”). Participating companies would be selected globally by public tender set up as a reverse auction (for example, maximum reservation fee for a platform technology would be € 2 Mio. per annum). Cumulatively, the companies chosen would cover all areas of product development necessary to fight pandemics (see chart below). At the discretion of EU HERA, such options could be made available to LMICs.
The options would be blended with venture debt, or classical loan funding for capacity builds by EU companies (“Investment Capital”). Such blend should be optional, and companies could opt out of the loan funding element at their discretion.
Since pandemics may start regionally as epidemics, the capacity options procured by the EU HERA under its Programme could be made available to
non-EU countries, especially LMICs, at the discretion of the EU.
EU Programme for Rapid Emergency Preparedness
The initiation of an EU Programme for Rapid Emergency Preparedness would encompass the following tasks:
- Running a public tender to select 16+ “best-in-class” companies globally;
- Procuring relevant capacity options from these companies by agreeing on an “emergency rate card”;
- Providing venture debt financing to those selected companies interested with the aim to close gaps in their current capacity offering;
- Managing the EU PREP Program.
WHAT IS HAPPENING NOW
On November 11, 2020, the European Commission has published a policy briefing on "Strengthening our medical and scientific agencies", under which a new EU health emergency preparedness and response authority is being outlined. A conclusive proposal has been announced for late 2021.